Neurodegenerative disease
Therapeutic target proteins associated with neurodegenerative disease exhibit intrinsic disorder, form multi-component complexes, or undergo aggregation, posing challenges to the investigation of their interactions with drug molecules and the underlying mechanisms. MDS makes analyzing such challenging targets straightforward as it works in solution and overcomes the limitations inherent to surface-based methods.
Overview
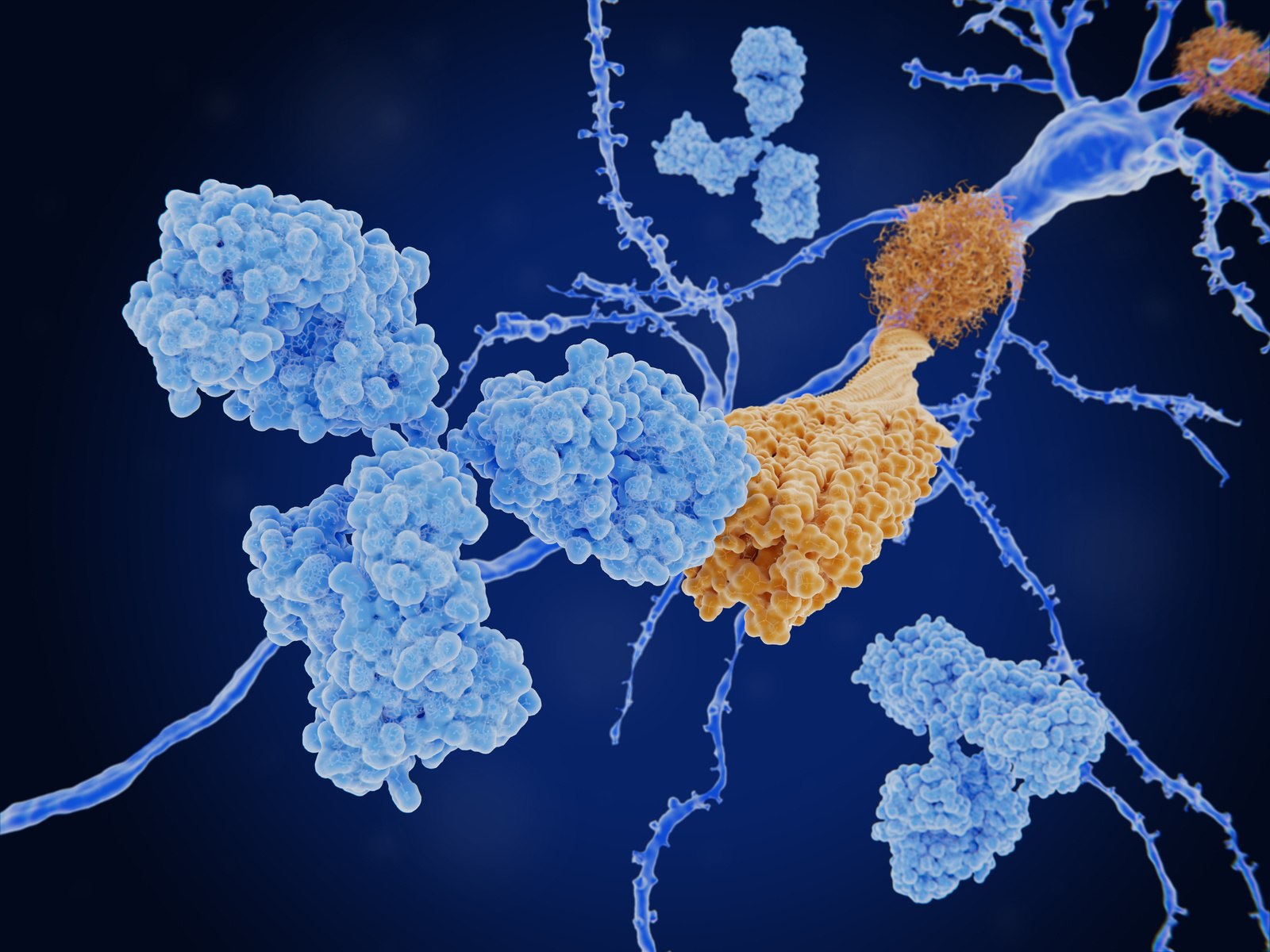
Neurodegenerative diseases (ND) affect millions of people worldwide and are characterized by the accumulation of neurotoxic aggregates in the brain leading to neuronal death. Immunotherapeutic approaches against NDs focus on antibodies that target the pathogenic aggregated forms of proteins such as α-synuclein or Aß peptide related to Parkinson’s disease and Alzheimer’s disease, respectively. ELISA and other surface-based methods are commonly used to identify antibodies that specifically target these pathogenic aggregates but tethering these sensitive proteins to a surface is often not feasible or induces artificial structures that do not occur in patients. Microfluidic diffusional sizing (MDS) avoids surface immobilization and thus preserves the disease-relevant structures of the aggregates which enables identification of antibodies that bind with high specificity and have the most effective mode of action against disease progression.
Key advantages of MDS
Case Study
Kinetic fingerprints differentiate the mechanisms of action of anti-Aβ antibodies.
Linse et al., Nature Struct. Mol. Biol., 2020, 27, 1125–1133. DOI: 10.1038/s41594-020-0505-6
During our research into protein amyloid formation, we have used many different techniques for assessing protein interactions – all with different limitations and advantages. With diffusional sizing, we were able to confidently generate accurate and complete in-solution data using amyloid proteins to connect stoichiometry and binding affinities with protein self-assembly. The data could provide key insights for novel therapeutic approaches in Alzheimer’s disease.
Get started
To study intrinsically-disordered or aggregated proteins in their native state in solution we recommend the following:
Workflow specification and benefits:
- 25 min run time
- KD range from pM to µM
- Provides stoichiometry
- Amount of antibody 1-10 µg (affinity dependent)
- Amount of oligomer or fibril 10-25µg
- 12 µL of sample per triplicate
- Use of complex background e.g. serum, CSF
- Quick & easy to perform